Explain Why Carbon-14 and Nitrogen-14 Are Not Considered Isotopes
Why is nitrogen 14 stable. The average atomic mass of a lead atom is 2072 amu.

Explain The Cleansing Action Of Soaps And Detergents A Plus Topper Soapsanddetergents In 2021 Detergent Soap Soap Detergents
Four isotopes of lead include lead-204 lead-206 lead-207 lead-208.

. Explain why carbon-14 and nitrogen-14 are not considered isotopes of each other. Explain why carbon-14 and nitrogen-14 are not considered isotopes of each other. Similarly how does nitrogen turn into carbon 14.
Since Carbon-14 and Nitrogen have different atomic numbers ie. Stages of Carbon-14 Formation Neutrons are ejected from nuclei of the upper atmosphere in collisions with cosmic rays A. There are always 7 protons because the nucleus is always a nitrogen nucleus Z7.
N-14 has an atomic mass of 14 and an atomic number of 7 so its number of neutrons is 7. Explain why carbon-14 and nitrogen-14 are not considered isotopes. Explain why carbon-14 and nitrogen-14 are not considered isotopes of each other.
Explain why carbon-14 and nitrogen-14 are not considered isotopes of each other. Same mass number but different atomic number. Since Carbon-14 and Nitrogen have.
Because they are two different elements. Because they are two different elements. Heres the answer Elements have the same atomic number not the same atomic mass.
Because they are two different elements. Because they are two different elements. C-14 therefore has 8 neutrons.
Same mass number but different atomic number. Elements with the same atomic number but different atomic masses are called isotopes. Explain why carbon-14 and nitrogen-14 are not considered isotopes.
They all have the same atomic number same number of protons. Muminat 10 months ago. Which isotope of lead is likely to be the most abundant.
Elements have the same atomic number not the same atomic mass. Same mass number but different atomic number. Terms in this set 14 isotope.
Elements with the same atomic number but different atomic masses are called isotopes. Some isotopes of nitrogen are unstable.

Doe Explains Isotopes Department Of Energy
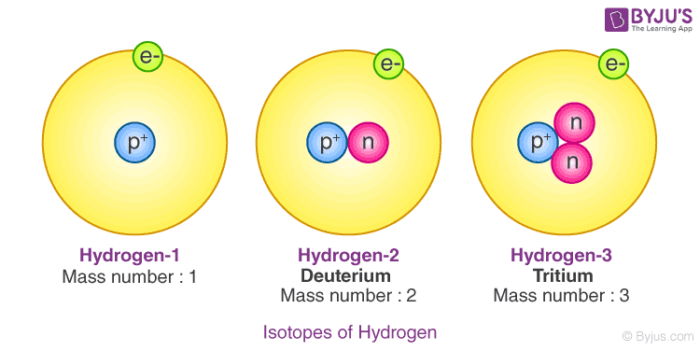
Isotopes Of Hydrogen Plutonium Deuterium Tritium With Examples Videos
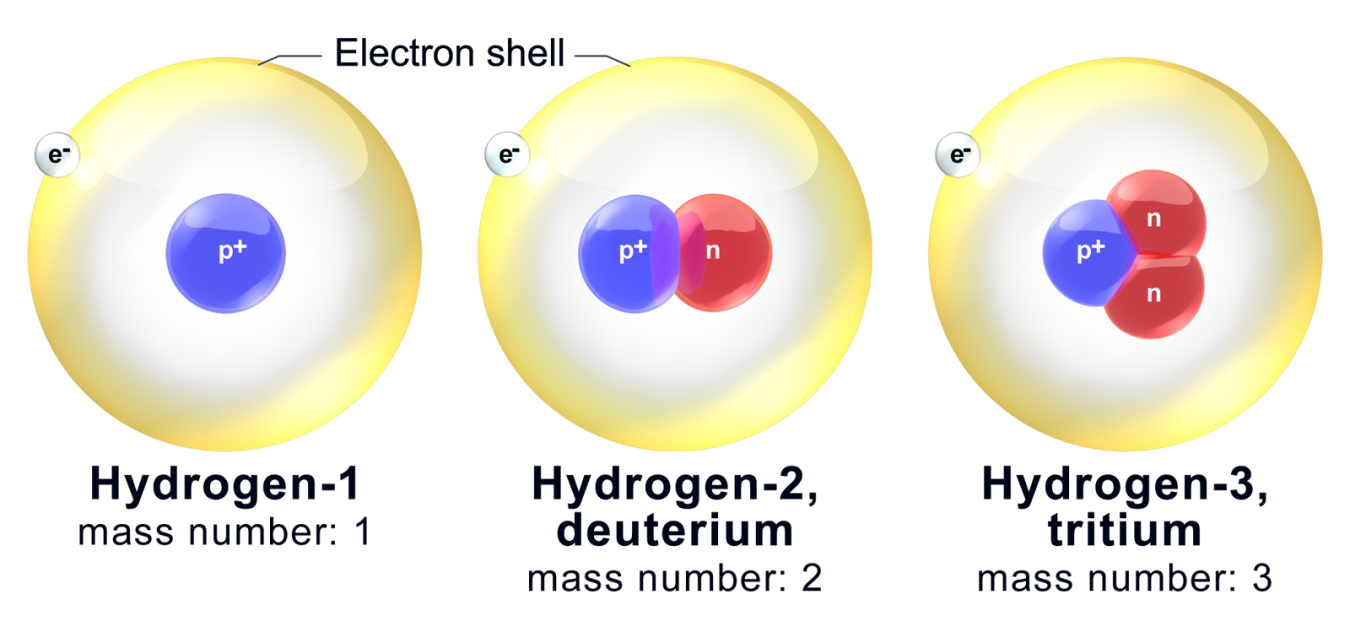
No comments for "Explain Why Carbon-14 and Nitrogen-14 Are Not Considered Isotopes"
Post a Comment